I did a lot of amateur research (amateur although strictly confined to industrial experts’ writings, not mumbo jumbo) into PC watercooling to try to figure out how to make a good coolant, since coolant makers offer voodoo products with ingredients that aren’t good (like dyes, fluorescents, and opacifiers) as well as trade secrecy. I discovered, for instance, that an industrial consultant for said glycols (whether ethylene or propylene) cannot be less than 20% of a water solution or they will develop harmful biological growth. The solution will become quite harmful to the metals it’s supposed to protect.
That contradicted an absolutely huge amount of amateur advice about “splashing” glycols in mixtures and using small percentages, like 5–10%. Apparently, too little glycol is worse than not adding it at all. That’s the opposite of the amateurs’ advice. They claimed that even this smaller amount of glycol will function as a biocide and/or increase lubrication and/or improve corrosion resistance. And, some commercial small business watercooling coolants claim to use less than 20% glycol, so they are apparently a bad choice for your equipment.
One of the things that I focused on a lot was on stopping the corrosion of copper. Since copper is commonly used in speaker parts (amp internals, wiring/connectors, etc.) I felt that I would share some of what I learned with the community here. Specifically, it’s about what can be done to treat the copper to reduce its penchant for corrosion due to air exposure. The short answer seems to be a dip in a tolytriazole solution (tolytriazole dissolved in ethanol).
However, other solutions may be even better. Some may be considered ‘good enough’ depending on the criteria. IBM uses BTA (benzotriazole) for its computer watercooling systems, probably because BTA is much more water-soluble than tolyltriazole. However, it may be more optimal to dip the copper parts in an ethanol solution of tolylriazole before putting it into a loop containing BTA water.
There was an interesting study involving the preservation of copper artifacts. Only one of the tested solutions stopped corrosion completely and it wasn’t one of the solutions using distilled water as the solvent:
BTA (benzotriazole) + AMT (1,3,4-thiadiazole) in ethanol
The ratio was .1M BTA + .01M AMT. Surprisingly, such a small amount of AMT did make a difference.
The same combination in distilled water had comparatively good performance (versus the other chemicals and mixes beyond the one cited above) but corrosion still happened. I believe that the artifacts were kept in the liquid. I do not know if the AMT, for instance, would make a difference for parts that are removed from the liquid. I also don't know how finger oils/contaminants will interact.
I also do not think that the study included tolyltriazole. Here is a link to the study:
A Corrosion Inhibitor Test for Copper-Based Artifacts on JSTOR
Tolyltriazole is apparently commonly used in industry, not only for some automotive corrosion resistance (like in some antifreeze mixtures) but also to protect copper parts from tarnishing from air exposure and such. As far as I know it is common for copper parts to be dipped in a solution of this.
advantage (versus benzotriazole) : thinner yet more effective coating (more hydrophobic) than benzotriazole
disadvantage: poor water solubility
There is also a longer carbon chain triazole. That is napthotriazole (related to napthalene — moth balls). It was briefly used in the laundry business. One piece of data I remember reading gave it better protective qualities than even tolyltriazole but I did not find any chemical companies that offer it. It would certainly not be soluble in water but if used with the dip method (ethanol?) might be better than tolyltriazole to coat copper to protect it from air exposure.
Copper oxides have very low thermal conductivity when compared with the copper metal, so a thin protective layer is better for PC heatsinks and watercooling waterblock channels than oxidized metal. I assume the electrical conductivity is also much lower.
(Despite the reduction in thermal conductivity and the problem of unsightly corrosion—that gets worse—many in the PC water-cooling community recommend using only distilled water with a silver coil as a biocide. That does not seem to be good advice due to the solution not protecting from galvanic corrosion, silver exacerbating it due to the presence of nickel in particular, there being no pH buffer, etc.)
Another mistaken bit of advice from amateurs (propped up by some small businesses) is to use silver as a biocide. For one thing, silver is not compatible with triazole inhibitors. It bonds with them, making both the silver and the triazole neutralized/worthless. Then, there is the issue of silver and galvanic corrosion.
Triazoles are rather stable in water and also harmful to aquatic life (not just people) so they shouldn’t be flushed down drains. They should be disposed of correctly as toxic waste. Their vapor pressures should be low enough for them to make evaporation of the solvent prior to disposal possible, for greater ease of disposal (particularly if the solvent in distilled water). You should also use protective equipment when dealing with them and all that. Research best practices if you handle them. They aren’t the sort of super-toxic stuff that needs a fume hood but you also don’t want to breathe the dust or spread it around. BTA is used in amateur and professional film photography. Handle with an appropriate level of care. Tolyltriazole may be less toxic since it’s much less soluble but I haven’t looked into that.
The copper artifact protection article said one of the goals was to find a less toxic alternative to triazoles. The result was not good for anything except a triazole.
(Just in case anyone is also doing something involving watercooling… do note that aluminum and copper do not play well together due to galvanic corrosion. Silver coils (as biocides) with typical nickle + copper parts also are a questionable combination. The easiest way to avoid galvanic corrosion is to use a single metal but in PC watercooling I’ve never found a single vendor selling copper fittings. They’re always nickle plated. There is also possibly a concern about the flux used. Also, various pH buffers like borate are often used to stabilize coolants so things aren’t always very simple.)
Copper has some wonderful qualities but resistance to corrosion is not one of them. I don’t know if there is an alloy that provides close to copper’s benefits without the ease of corrosion but I doubt it. Gold certainly isn’t a good alternative for electrical conductivity but it is great for corrosion resistance (and thus could be considered ‘good enough’ for conductivity since it’s so stable versus corrosion that engineers can build specs around it more reliably). Yes, I know that pure gold isn’t an alloy. I don’t know what industry is using to reduce silver’s tarnishing. Perhaps it’s tolyltriazole as well, or some sort of alloy. I haven’t investigated silver cables, connectors, and things of that sort.
One basic point of this topic is to point out that copper should be, at least, dipped in a protective triazole for most purposes — particularly if not protected by some kind of visible coating. And, even then it’s probably best to have it protected inside of that coating unless air can be permanently evacuated and replaced with something inert like argon.
I have read that the Magnepan Tympani III uses copper wire and that Magnepan doesn't replace corroded copper wire when refurbishing that speaker for customers. Instead, the panels are replaced with aluminum wire. I have also read that Quad ESLs have used copper wire. So, protection of electrostat wires from corrosion seems to be important if one plans to use copper, particularly for refurbishing according to original specs. Hopefully this post will incite people who might otherwise just use bare wire and hope for the best to consider the possibility that some sort of corrosion protection might be in order, depending upon the situation.









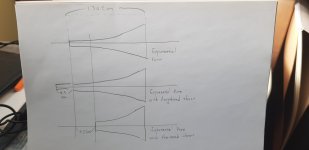

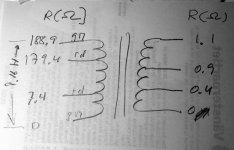




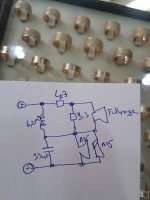
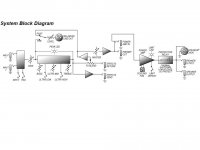



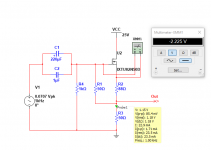
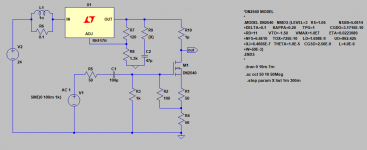
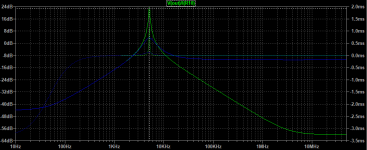
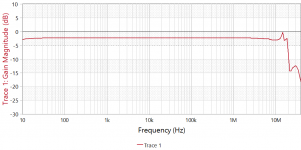
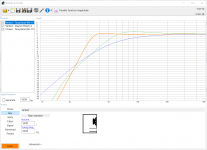










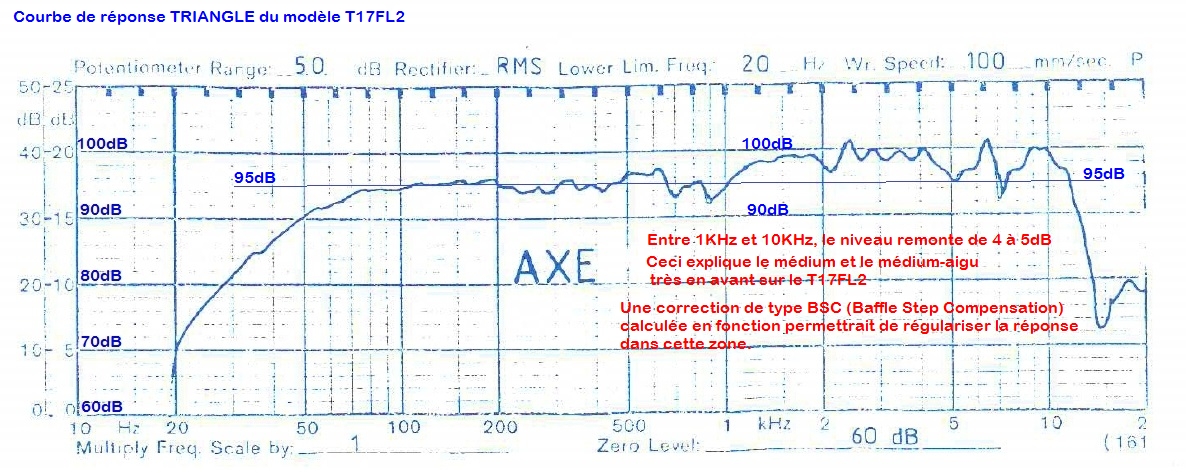
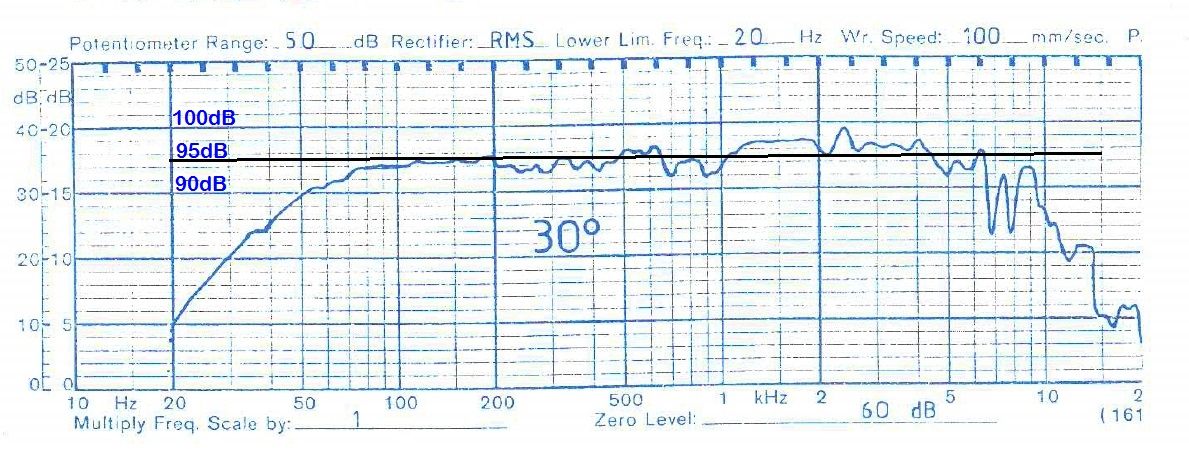


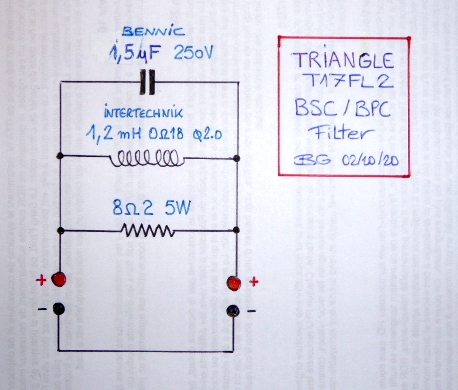
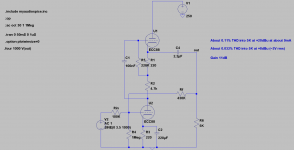
 - I decided to correct that issue by experimenting the principle of a Baffle Step Compensation crossover... Not trivial for a full-range speaker !
- I decided to correct that issue by experimenting the principle of a Baffle Step Compensation crossover... Not trivial for a full-range speaker !