Tubes are not always in equilibrium in any case.
Nothing like a good nights sleep to get a clearer picture:
the only time that a valve is in equilibrium is when it is not in use. I should have seen that before

When it is turned on, there are temperature differences between the surfaces of the elements and glass which means that there are different vapour pressures at different locations.
In other words, the Ba vapour pressure close to the cathode is higher than that of the cooler glass or grid, resulting in diffusion of barium atoms away from hot surfaces.
Now with the evaporation of bariumoxide things get a little more interesting.
Bariumoxide is a salt and the formula BaO doesn't mean that it consists of Ba-O particles, it just means that it is made up of the same number of barium ions as there are oxygen ions.
So what happens when a salt evaporates? It releases the individual ions: Ba2+ and O2-. The positive barium atoms will be attracted to the closest negative surface: the control grid. At the grid it will absorb two electrons and return to its metallic state (or possibly form an auride).
The negative oxygen ions are accelerated to positive electrodes, where it could form a new metal oxide, possibly hitting another oxide recombining with that to an oxygen gas molecule.
Really ? No, can't find it so far. A.G Naumovets presumably. Any pointers ?
Drop me an email, I'll see if I can get you a copy.
A tube thoroughly warmed up and running continuously also is in equilibrium, but a different sort of equilibrium.Nothing like a good nights sleep to get a clearer picture:
the only time that a valve is in equilibrium is when it is not in use. I should have seen that before
More correctly, the pressure everywhere in the tube is the same. At the cathode, the Ba vapor pressure is just a tiny bit above the tube internal pressure, and so there is a net evaporation of Ba. The grid is cooler, so the Ba vapour pressure there is lower than the tube internal pressure, causing the Ba to precipitate on the grid.In other words, the Ba vapour pressure close to the cathode is higher than that of the cooler glass or grid, resulting in diffusion of barium atoms away from hot surfaces.
The Ba-O can exist in particles ranging from those visible by microscope, down to just a BaO molecule.Now with the evaporation of bariumoxide things get a little more interesting.
Bariumoxide is a salt and the formula BaO doesn't mean that it consists of Ba-O particles, it just means that it is made up of the same number of barium ions as there are oxygen ions.
That is getting outside my knowlege - I am an electrical engineer, not a chemist. Is it in fact the only way it works? Cannot sublimation of BaO occur? Handbooks give the heat of sublimation for BaO at 101 kcal/mole. What does this refer to - conversion to gaseous BaO or conversion to Ba++ & O--?So what happens when a salt evaporates? It releases the individual ions: Ba2+ and O2-. The positive barium atoms will be attracted to the closest negative surface: the control grid. At the grid it will absorb two electrons and return to its metallic state (or possibly form an auride).
Which would mean most would go to the anode (all if it is a triode). Yet tube books say that the gettering has an ongoing role in taking up oxygen.The negative oxygen ions are accelerated to positive electrodes.......
That is true for solids.The Ba-O can exist in particles ranging from those visible by microscope, down to just a BaO molecule.
Bariumoxide is a salt. In a salt there are no molecules, only ions held together by electrostatic forces. No barium ion belongs exclusively to a specific oxygen ion (contrary to molecules like carbondioxide where an individual carbon atom and two oxygen atoms are bonded together in a single molecule).
In the gas phase salts are dissociated into the individual ions, just like in solutions.
Salts can be sublimated to a different location. The gaseous ions diffuse to a cooler place and condensate together.That is getting outside my knowlege - I am an electrical engineer, not a chemist. Is it in fact the only way it works? Cannot sublimation of BaO occur? Handbooks give the heat of sublimation for BaO at 101 kcal/mole. What does this refer to - conversion to gaseous BaO or conversion to Ba++ & O--?
Reminds me of the purification step I once did in a chromium(III)chloride synthesis: heat up the raw CrCl3 in a glass tube and watch the pure crystals grow on the other side. It had to be done in a nitrogen atmosphere as Cr3+(g) is not stable in air. The solid CrCl3 salt is stable in air. (and quite beautiful)
In the presence of an electrical field however, gaseous cations and anions won't migrate together as the field would push them in opposite directions.
Which would mean most would go to the anode (all if it is a triode). Yet tube books say that the gettering has an ongoing role in taking up oxygen.
True, the getter is there to capture gasses.
Break the glass and you see the silvery metal turn white as it reacts with oxygen to form bariumoxide.
In a worn out valve you usually don't see the getter having turned white: it just has faded away. That means that there is no mentionable oxide deposit there.
I image this is because the little barium vapour in the valve reacts with gasses like oxygen forming solid salts. The vapour pressure is maintained by the evaporation of fresh barium from the getter flash, hence the slow fading.
This is also one of the reasons why the getter needs to be relatively hot: to ensure enough barium evaporation as the barium gas is used up.
The other reason being the reaction between barium and nitrogen. Bariumnitride is only formed at high temperature. I think it also needs an excess of barium, so the reaction takes place at the getter flash, not in the gas phase. (Not sure about this 'though.)
Note that barium is just one of many substances used for getter flash, one of the earlier ones, although favoured by RCA for power tubes up until the 1960's, and barium was often a component along with other elements.
Magnesium was pretty common.
The common ring getters used in the 1960's and later usually had a barium/aluminum/nickel powder mix in about equal quantities.
Small tubes for low level stages and battery tubes that don't heat the glass much usually had cerium+thorium getter flash, as it is said to be effective at much lower temperatures, even working well at room temperature.
Reference: Thomas C H, Getters, RCA 1966.
Magnesium was pretty common.
The common ring getters used in the 1960's and later usually had a barium/aluminum/nickel powder mix in about equal quantities.
Small tubes for low level stages and battery tubes that don't heat the glass much usually had cerium+thorium getter flash, as it is said to be effective at much lower temperatures, even working well at room temperature.
Reference: Thomas C H, Getters, RCA 1966.
Just to clarify, as this goes against what Sy (who claims to be a chemist, although on the internet nobody knows you are a dog as the saying goes) and Lucky have been saying about BaO evaporating & landing on the grid:In the gas phase salts are dissociated into the individual ions, just like in solutions.
...
Salts can be sublimated to a different location. The gaseous ions diffuse to a cooler place and condensate together.
In the presence of an electrical field however, gaseous cations and anions won't migrate together as the field would push them in opposite directions.
I think you are trying to say that Ba-O cannot exist in the gas phase. Solid Ba-O, being a salt, sublimates only into Ba++ and O-- ions
Since the electric field (grid is negative) sends them off in different directions, only Ba++ can end up on the grid. It may chemically react in the grid, or it may just seize 2 electrons (the grid is negative after all - hmmmm... I smell a rat here - just not sure what sort of rat it is.)
To get to the grid, Ba++ has to pass through the space charge - so it can sieze 2 electrons there. The O-- would be repelled back to the cathode by the space charge - hmmmm..... another rat.
Handbooks give the heat of sublimation for BaO at 101 kcal/mole. What does this refer to - conversion to gaseous BaO or conversion to Ba++ & O--?
Reminds me of the purification step I once did in a chromium(III)chloride synthesis: heat up the raw CrCl3 in a glass tube and watch the pure crystals grow on the other side. It had to be done in a nitrogen atmosphere as Cr3+(g) is not stable in air. The solid CrCl3 salt is stable in air. (and quite beautiful)
Bright purple, if I recall correctly. I've done that reaction as an intermediate to prepare some imine complexes, though we did it under vacuum rather than inert gas. It has to be kept very dry.
Hmmm:In the gas phase salts are dissociated into the individual ions, just like in solutions.
"BaO is a stable molecule even in the gaseous phase."
Encyclopedia of the Alkaline Earth Compounds, Richard C. Ropp 2012
Though I'm not a chemist, surely this says that the gas phase comprises associated Ba-O molecules or groups thereof?
Chemistry make my head hurt......
Hmmm:
"BaO is a stable molecule even in the gaseous phase."
Encyclopedia of the Alkaline Earth Compounds, Richard C. Ropp 2012
Though I'm not a chemist, surely this says that the gas phase comprises associated Ba-O molecules or groups thereof?
Chemistry make my head hurt......
Hmmm:
High-Temperature Chemistry of Silicates and Other Oxide Systems, N.A. Toropov (2012), page 145:
In contrast to all the other oxides of alkaline earth elements, barium oxide evaporates predominantly in the form of BaO molecules. Moreover, surprisingly, the following complex gaseous compounds of barium and oxygen are formed: Ba2O, Ba2O2, BaO, and Ba2O3.
This information was taken from a publication of mass spectrometric analyses in 1955.
So indeed, the vapour of BaO consists mainly of neutral particles. I didn't expect that.
I'm very curious to what SY will find on the grids of new and used valves.
Perhaps extend this work to non-gold grids as well? To assess the differences in deposits between regular and gold plated grids.
PS
The more common name for a hurting head as a result of (bio)chemical compounds is a hangover

High-Temperature Chemistry of Silicates and Other Oxide Systems, N.A. Toropov (2012), page 145:
In contrast to all the other oxides of alkaline earth elements, barium oxide evaporates predominantly in the form of BaO molecules. Moreover, surprisingly, the following complex gaseous compounds of barium and oxygen are formed: Ba2O, Ba2O2, BaO, and Ba2O3.
This information was taken from a publication of mass spectrometric analyses in 1955.
I'm not able to acess this textbook.
I assume you mean the 1966 or 1969 edition. I cannot see that there would be a 2012 version - the principal author died in 1968.
So what was the 1955 publication that the information was taken from? I would like to check that.
I'm guessing that the sample(s) were obtained under conditions not applicable to vacuum tubes. Most likely the solids were not activated, which produces free Ba atoms diffused through the matrix. Once Ba atoms exist, they should comprise practically all the vapour emitted (apart from the oxygen), as the vapour pressure of Ba is 10^9 times that of BaO at applicable temperatures.
Incidentally, no Ba vapour will come from the getter flash as you stated. The glass envelope is seldom above 450 K. At that temperature the vapour pressure is about 10^-11 what it is at cathode temperature (1050 k). Instead, any Ba emitted from the cathode that doesn't get captured by tube electrodes will actually add mass to the getter flash.
Give your head another bang, Popilin - the electrons within a conductor are not affected by the electric field outside it.
According to the free electron model, the electrons producing the work function barrier are outside the cathode, and then you have no excuse to admit Schottky effect in conductors.
Your bricks evidently are not hard enough - try a piece of Krupp cemented armour.
I discovered a new brick, extremely hard, I call this Keitptonite. 😀
Obviously, the accelerating electric potential, Va must be referred to the emitter surface…..
Ah, at last. Your English is a little imprecise - do you mean Va is the voltage at the cathode surface?
….. now if the emitter surface is a valve cathode surface; Va becomes the electric potential of the anode with respect to the cathode, aka anode voltage, as you clearly understood a long ago
No, that's incorrect. The anode voltage is not the voltage at the emission surface.
If there is any possibility to ruin a definition, you surely will find it. 🙄
You confuse potential with voltage, and finally no, we do not need the voltage at the emitter surface; all that we need is an external electric field, or even easier Va.
E = - ∇ φ = - dφ/dx
Integrating
E dak = [φ(a) – φ(k)] = Va
Then
E = Va/dak
Notation is self explanatory.
The voltage of the emission surface may be millivolts or less above the cathode nickel sleeve.
Silly question: How do you measure it?
With your little gopher perhaps? 😀
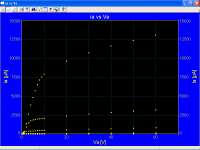
Seems you are the guilty one there. A glance at the pure tungsten type datasheets show the Ia vs Va line is practically horizontal once saturatioj is past, varing only a few percent attributable to end effects & such.
As you did not understand the scale factor issue, you also do not understand that Ia vs Va is the wrong plot in order to search for Schottky effect, as I said before, you must plot Ln(ia) vs √Va
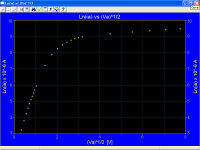
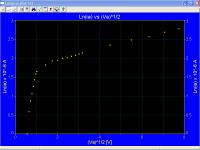
Measured ia vs Va for an oxide coated cathode, for low cathode temperatures, also look practically horizontal, however the correct plots Ln(ia) vs √Va look like as they should be
Five plots ia vs Va
Ln(ia) vs √Va corresponding with the previous first plot at the top
Ln(ia) vs √Va corresponding with the previous last plot at the bottom
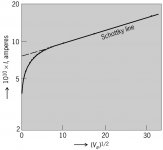
Surely you will complain about that, so I will repeat the plot for a tungsten cathode
Logarithm of thermionic emission current I of tungsten as function of square root of anode voltage Va. (After W. B. Nottingham, Phys. Rev., 58:927–928, 1940)
Attachments
I'm not able to acess this textbook.
I assume you mean the 1966 or 1969 edition. I cannot see that there would be a 2012 version - the principal author died in 1968.
So what was the 1955 publication that the information was taken from? I would like to check that.
I'm guessing that the sample(s) were obtained under conditions not applicable to vacuum tubes. Most likely the solids were not activated, which produces free Ba atoms diffused through the matrix. Once Ba atoms exist, they should comprise practically all the vapour emitted (apart from the oxygen), as the vapour pressure of Ba is 10^9 times that of BaO at applicable temperatures.
2012 must be the year Springer Science and Business Media reprinted it.
The preview is available on google books:
https://books.google.nl/books?id=0wj3BwAAQBAJ&dq=bao+vapor&source=gbs_navlinks_s
Incidentally, no Ba vapour will come from the getter flash as you stated. The glass envelope is seldom above 450 K. At that temperature the vapour pressure is about 10^-11 what it is at cathode temperature (1050 k). Instead, any Ba emitted from the cathode that doesn't get captured by tube electrodes will actually add mass to the getter flash.
Over use the getter flash fades away. How can this happen if not evaporation?
Over use the getter flash fades away. How can this happen if not evaporation?
Perhaps reaction?
Chemistry make my head hurt......
I never did like chemistry.
If you take any piece of electronic equipment, then no matter what it is, if you drill down into it, you end up with these fundamental electronic components:-
Resistors
Capacitors
Inductors
Transformers
Gyrators
Voltage controlled current sources.
Each of these is well defined and every electronic tech or engineer knows what they are. They are very simple. Understand these 6 things and you can understand everything in electronics.
Each is something you can hold in your hand. They all have some "strays" eg practical resistors have a bit of C and a bit of L, but they all do just what their names say they do.
You can analyse an ordinary amplifier circuit and calculate its performance in seconds.
In contrast, the further you drill down in chemistry looking for fundamentals, the more complex it gets, until you end up with string theory - ugh! The periodic table, much loved by science teachers, ain't (when you look at in detail) periodic - it's really a matter of general trends with lots of exceptions. And many gaps in what's known eg Osmium: melts at 3306 K. So what are its transport properties above 1800 K? Nobody knows.
Even to do a "simple" thing like calculate the reaction rate and products of hydrogen and oxygen at any temperature takes a powerfull computer grinding away for days. Weeks if it is a PC.
I'd rather take up psychiatry than chemistry. That's a field in which the fundamentals are mostly completely unknown (classical & operant conditioning being notable exceptions), but submit a Ph.D thesis on something bizare, eg the statistical realtionship between choice of toothpaste and preferred sexual position, act as resident at a drug treatment centre for 2 years prescribing drugs for incurable drug addicts and you're in!
Last edited:
I discovered a new brick, extremely hard, I call this Keitptonite.
Give it a try then.
If you read up on the subject, you'll see that researchers have used such things as special custom test vacuum tubes with essentially two oxide cathodes pressed together emission surface to emission surface, enabling them to measure the radial resistance of the activated oxide. If you have a current flowing in a resistance, then you have a voltage drop.Silly question: How do you measure it (volatge at emission surface)?
With your little gopher perhaps? 😀
Why do you keep up with this nonsense?As you did not understand the scale factor issue, you also do not understand that Ia vs Va is the wrong plot in order to search for Schottky effect, as I said before, you must plot Ln(ia) vs √Va.......
{lots of graphs of the same thing as before}
As I pointed out before, it matters not a whit what scales you choose to graph with, or whether the axes be log, lin, square root, or freddy frogs.
What matters is the percentage change in Ia for a given range in Va. Or, if you like, the percentage change on Ln(Ia) for a given range of root Va.
And as I pointed out to you before, your monochrome graph puporting to be for a pure tungsten cathode simply isn't. There's way too much change in Ia when compared with measured data for actual pure cathode tubes such a the AV44 regulator tube.
If you vary the cathode temperature, then the saturation curent & voltage changes, the magnitude of the saturation currents change, but the shape essentially does not, the percentage change does not change, and that's what the data on real tubes shows.
Now, consider this: Assume a planar diode (that is a diode with flat cathode near a flat anode). Let's say the cathode-anode spacing is 5mm. Let's say the anode voltage is +100 V. The electric field is thus 20V/mm - we can assume the space charge is well depleted. The anode current will be whatever it is, as a function of the tube dimensions, cathode temperature, schottky effect.
Now, increase the anode-cathode spacing to 20 mm and the anode voltage to +400V. The electric field is still 20V/mm.
According to you, the current will be greater, as you think schottky effect is driven by anode voltage. It ain't so - the current will be the same as before.
Don't just tap your head against that Keitponite - get well back, and take a run toward it, screaming "Aaagggghhhhh!"
Then, with that planar diode with 20mm cathode-anode spacing, insert a grid exactly half way between the cathode and the anode. Put +200V on it. The electric field everywhere inside the tube structure is the same as before - a nice even 20V per mm. The grid will take some curent from the anode, but that doen't matter. Which are you going to regard as the Va for estimating schottky effect - +200V on the grid, or +400V on the anode? Think about it and what it means.
Give your skull a real bang now.
Stop posting multiple graphs of the same thing. We've seen them. Repetition does not prove anything.
Last edited:
2012 must be the year Springer Science and Business Media reprinted it.
The preview is available on google books:
https://books.google.nl/books?id=0wj3BwAAQBAJ&dq=bao+vapor&source=gbs_navlinks_s
Yes, I know its on Google books. And I see from the title page that it's the 1966 edition. But the site won't let me look at page you cited.
Please post the 1955 source of the data citation, so I and anyone else can look that up.
If you take any piece of electronic equipment, then no matter what it is, if you drill down into it, you end up with these fundamental electronic components:-
Resistors
Capacitors
Inductors
Transformers
Gyrators
Voltage controlled current sources.
Each of these is well defined and every electronic tech or engineer knows what they are. They are very simple. Understand these 6 things and you can understand everything in electronics.
That explains a lot; maybe you must add “Valves” to your list.
That explains a lot; maybe you must add “Valves” to your list.
Nope.
Valves are voltage controlled current sources, already in the list.
Nope.
Valves are voltage controlled current sources, already in the list.
Bingo!
Your exotic planar diode with variable geometry of post#295 it is not...

- Status
- Not open for further replies.
- Home
- Amplifiers
- Tubes / Valves
- Why Gold Grids