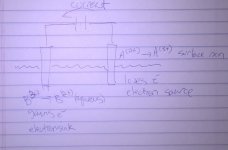
OK, I might have figured it out... the electrons flows to the metal reducing it , which means they must come from somewhere, in this case the surface of the reference electrode.
So electrons are shuffled around, without a "loop" but still keeping charge conserved.
So electrons are shuffled around, without a "loop" but still keeping charge conserved.
current flow requires a loop. That is what "circuit" means. A route for current to flow around. All the current leaving a Source MUST RETURN to that Source. That is a LOOP !
current flow requires a loop. That is what "circuit" means. A route for current to flow around. All the current leaving a Source MUST RETURN to that Source. That is a LOOP !
I understand the loop rule in circuits where there is a return path. But look at it like this:
A metal goes from M3+ to M2+ by gaining an electron. That electron is now in the ion and accounted for. What you are proposing means that 100% of current goes back to the reference electrode through the solution. But how do you account for the new electron in the metal? If 100% of current goes back, and the metal still gets an extra electron, you are creating electrons.
No you don't. You only understand the rule when you realise that there are no exceptions - except the trivial exception of electrostatics.vladimirb0b said:I understand the loop rule in circuits where there is a return path.
A battery, whether charging or discharging, is a normal circuit element and so fully subject to Kirchoff's laws. You push electrons into one terminal and the same number of electrons comes out of the other terminal; the circuit insists on that. You need to start from that fact, and then work out whatever electrochemistry is consistent with that. Don't try to start with the chemistry, as chemistry is fully subject to the laws of physics. You don't have isolated electrodes in a solution; you have two electrodes in a circuit.
I now understand how the battery doesn't contradict Kirchoff.
People keep saying there has to be a loop but won't tell me what it is. Can you directly refute my reasoning, particularly the second of those quotes? I am trying as hard as I can to find a proper 100% loop that can still change the charge of an ion.
the electrons flows to the metal reducing it , which means they must come from somewhere, in this case the surface of the reference electrode.
So electrons are shuffled around, without a "loop" but still keeping charge conserved.
A metal goes from M3+ to M2+ by gaining an electron. That electron is now in the ion and accounted for. What you are proposing means that 100% of current goes back to the reference electrode through the solution. But how do you account for the new electron in the metal? If 100% of current goes back, and the metal still gets an extra electron, you are creating electrons.
People keep saying there has to be a loop but won't tell me what it is. Can you directly refute my reasoning, particularly the second of those quotes? I am trying as hard as I can to find a proper 100% loop that can still change the charge of an ion.
I understand the loop rule in circuits where there is a return path. But look at it like this:
.
There is always a return path, otherwise there is no current flow, just electrostatic waves if there is a potential difference between two points.
I'm not a chemist, much like I'm not an electrician.
But here goes.
Charge (or battery voltage) is an accumulation of opposite charges either side of a boundary. Those charges came from within the source (battery or capacitor) that was one half of the circuit. The charges initially moved to create the voltage/charge.
Connect an external circuit and the current will flow. It flows from the source and returns to the source. That flow and return is around the external circuit and the internal circuit. There's your loop/circuit.
But here goes.
Charge (or battery voltage) is an accumulation of opposite charges either side of a boundary. Those charges came from within the source (battery or capacitor) that was one half of the circuit. The charges initially moved to create the voltage/charge.
Connect an external circuit and the current will flow. It flows from the source and returns to the source. That flow and return is around the external circuit and the internal circuit. There's your loop/circuit.
To Quote Ralf Morrison, from "THE FIELDS OF ELECTRONICS"
Kirchoffs circuit laws...
https://en.wikipedia.org/wiki/Kirchhoff's_circuit_laws
Energy can be stored chemically. When there is a chemical reaction, energy
is released. In an explosion, this energy can be released as heat, light, and
mechanical motion. In some arrangements, chemical energy can be released
electrically. A battery is an arrangement of chemicals that react when the active
components are allowed to circulate their electrons in an external circuit. The
energy that is stored chemically is potential energy that is available to do
electrical work. In rechargeable batteries the chemistry is reversible and energy
can be put back into the battery.
The terminals of the battery present a voltage to the world. This is electrical
pressure trying to move electrons so that the chemicals in the battery can
attain a lower energy state. This is analogous to water pressure in a water tank
where the water is trying to get to a lower energy state. This water pressure
is no different from the voltage between two oppositely charged conductors
in space. There is an E field between the terminals of the battery. If this is a
12-V battery, it takes 12 V of work to move a unit of charge between the two
terminals. This work is independent of the path taken by the test charge. This
includes a path through the heart of the battery. The E field cannot be seen,
but it is there. This field extends right into the battery, where the atoms are
under pressure to release their external electrons.
In Figure 1.2 the static charges can be removed and the E field disappears.
In the case of the battery, the E field and the associated charges on the conductors
will persist until the battery is dead. When charge is allowed to flow
through a circuit connected to the terminals, the battery replaces this charge
and maintains the electrical pressure. A battery is thus a voltage source that
does not sag. It is like being connected to the city water supply. No matter
how much water you draw, the water pressure is the same.
Kirchoffs circuit laws...
https://en.wikipedia.org/wiki/Kirchhoff's_circuit_laws
I have a grasp now of the battery charge and discharge. What I am talking about is aqueous electrochemistry. Way off topic, I know, but might as well try again.
I have attached a picture of the setup in question as I understand it now. I mislabeled the ion charges but it makes no difference for the example. Please let me know where the loop is.
If there is no reaction at the right side electrode, charge conservation is not possible via the reasoning a few posts back about creating electrons
If you add a ground along with or instead of the right side electrode, the charge conservation problem still exists
I have attached a picture of the setup in question as I understand it now. I mislabeled the ion charges but it makes no difference for the example. Please let me know where the loop is.
If there is no reaction at the right side electrode, charge conservation is not possible via the reasoning a few posts back about creating electrons
If you add a ground along with or instead of the right side electrode, the charge conservation problem still exists
Attachments
Last edited:
I have a grasp now of the battery charge and discharge. What I am talking about is aqueous electrochemistry. Way off topic, I know, but might as well try again.
Before trying again and dragging this even further off topic, get a basic freshman chem book, read carefully the chapters on electrochemistry, and work the problems.
OK, I can put it back on topic 🙂 Vaguely remember someone posting about this but not going to read the whole thread.
I bought two of these: https://www.amazon.com/gp/product/B002IWEDSS/
There should be enough room there to cut any wires that need to be cut.
I bought two of these: https://www.amazon.com/gp/product/B002IWEDSS/
There should be enough room there to cut any wires that need to be cut.
Is it just me? I seem to have lost the plot/point of this thread... Though it is entertaining. Still wondering where Max's flash memory tangent was supposed to be leading us... 😀
USB Data cable -> USB Cable quality -> USB Noise affecting analog sound -> USB Filters -> Jitterbug is BS -> EVS and battery ground tweak -> battery chemistry fail -> electrochemistry fail
Am I missing anything?😀😀😀
Am I missing anything?😀😀😀
Is it just me? I seem to have lost the plot/point of this thread... Though it is entertaining. Still wondering where Max's flash memory tangent was supposed to be leading us... 😀
A long winding road similar to Dantes' epic adventure, where bit identical files sound different due to some reason, one is stored on HD compared to stored on SSD, my favourite was files ripped using a linear PSU sounded better than same files ripped using a SMPS. We have had many a happy discussion around the same theme.
USB Data cable -> USB Cable quality -> USB Noise affecting analog sound -> USB Filters -> Jitterbug is BS -> EVS and battery ground tweak -> battery chemistry fail -> electrochemistry fail
Am I missing anything?😀😀😀
Err... Common sense..? 🙂
A long winding road similar to Dantes' epic adventure, where bit identical files sound different due to some reason, one is stored on HD compared to stored on SSD, my favourite was files ripped using a linear PSU sounded better than same files ripped using a SMPS. We have had many a happy discussion around the same theme.
😀 Good comparison!
Post191.
I can see a loop in the diagram.
Can anyone else see a loop, or is it in my imagination?
I can see a loop in the diagram.
Can anyone else see a loop, or is it in my imagination?
I can see a loop too. Maybe we are both imagining it?
Would this be a good time to draw people's attention to so-called displacement current? In some senses what happens inside a battery is not that different from what happens between the plates of a capacitor. In both cases dogmatic naive people may claim there is no current, but in reality the definition of current has been extended beyond mere flow of charges.
We are getting into one of those arguments where someone asks a question but doesn't know enough to understand the right answer so rejects it in favour of the wrong answer. These often arise when people try to understand complex things (like battery insides) before they have fully grasped (and accepted) simple things (like circuits). This discussion about batteries is incidental to the main point about the foolishness of adding random bits of conductor to audio grounds; I guess that arises from an inability to grasp the nature of potential difference (if only we always called it that, instead of voltage, much of the confusion would disappear) and the purpose of a 'ground'.
Would this be a good time to draw people's attention to so-called displacement current? In some senses what happens inside a battery is not that different from what happens between the plates of a capacitor. In both cases dogmatic naive people may claim there is no current, but in reality the definition of current has been extended beyond mere flow of charges.
We are getting into one of those arguments where someone asks a question but doesn't know enough to understand the right answer so rejects it in favour of the wrong answer. These often arise when people try to understand complex things (like battery insides) before they have fully grasped (and accepted) simple things (like circuits). This discussion about batteries is incidental to the main point about the foolishness of adding random bits of conductor to audio grounds; I guess that arises from an inability to grasp the nature of potential difference (if only we always called it that, instead of voltage, much of the confusion would disappear) and the purpose of a 'ground'.
An audiophile could waste all their free time auditioning the countless hi-fi products that claim this or that.Risking flame bait again here but how much would it hurt (anyone) to try an EVS? They have a 30 day return policy so you risk about $15 in shipping.
A better plan would be to only look a products that measure well or pass blind tests.
- Status
- Not open for further replies.
- Home
- Member Areas
- The Lounge
- Making a usb cable _ data only