The "orbital representation" tells us that the single electron in the hydrogen atom does not spread out uniformly, but follows probability density distribution patterns called orbitals (not to be confused with the orbits of the Bohr model). The specific pattern of an orbital depends on the energy state of the hydrogen atom.So how does a single electron occupy the entire space of a Hydrogen atom? Is it because the electrons determine size and hence there is simply no room left? How do you visualize this? We went from the classic criss cross orbital representation to the planar position to ?
"How" does the single electron occupy the entire space of a hydrogen atom? It does so by not being a solid particle, but by being a 'wave function' - diifficult to visualise I know, but that's where we are at!
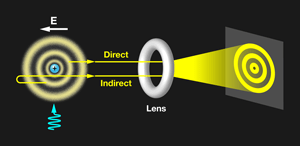
Physicists have claimed to have directly observed the electron orbital of a hydrogen atom using a "photoionisation microscope":
https://physics.aps.org/articles/v6/58
Attachments
Infinite compression, infinit expansion, infinite compression... yepp - like it! ;-)Yes he is! I recently attended (online as it was sold out) a talk by him at Southampton University (UK), this was his lovely slide demonstrating the idea
//
I think that there is enough evidence around us that there are other universes parallel to this one, its just that we mostly do not pay or do not want to pay attention to them.
Things like the deja-vu or Schrödinger's cat? I know it is very relative but I do think there is something about it...
Things like the deja-vu or Schrödinger's cat? I know it is very relative but I do think there is something about it...
Fascinating article. Pity there weren’t any pictures of the images they took. I remember seeing an electron microscope photo of the individual atoms on the tip of a pin - that pic was taken in the early 1960’s.The "orbital representation" tells us that the single electron in the hydrogen atom does not spread out uniformly, but follows probability density distribution patterns called orbitals (not to be confused with the orbits of the Bohr model). The specific pattern of an orbital depends on the energy state of the hydrogen atom.
"How" does the single electron occupy the entire space of a hydrogen atom? It does so by not being a solid particle, but by being a 'wave function' - diifficult to visualise I know, but that's where we are at!
Physicists have claimed to have directly observed the electron orbital of a hydrogen atom using a "photoionisation microscope":
https://physics.aps.org/articles/v6/58
Not parallel universe . higher dimensions
That the electron is simultaneously everywhere in its orbital. As we are in 3d space we only can observe the 3d electron . But actually its a 4d object .
That the electron is simultaneously everywhere in its orbital. As we are in 3d space we only can observe the 3d electron . But actually its a 4d object .
I've found a Wikipedia article that describes the photoionisation technique: https://en.wikipedia.org/wiki/Quantum_microscopyFascinating article. Pity there weren’t any pictures of the images they took.
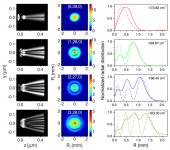
What is seen is an interference pattern, as explained in the following quote from the article:
The electron wave ends up producing an interference pattern because the portion of the wave directed towards the 2D detector interferes with the portion directed away from the detector. This interference pattern shows a number of nodes that is consistent with the nodal structure of the Hydrogen atom orbital.
There's more information and images here: https://physicsworld.com/a/quantum-microscope-peers-into-the-hydrogen-atom/
Attachments
Thanks for that Galu. Fascinating how the electron occupies certain bands based upon its excitation state - and the fact that what was predicted with match is now observed.
Yes, it's remarkable that the experimentally observed interference patterns (solid lines) compare well with the theoretically calculated probability distributions (dotted lines) - as seen in the graphs on the right hand side of the image I attached.
The link I gave is dated 2013, so I must investigate what further developments have been made.
The link I gave is dated 2013, so I must investigate what further developments have been made.
My phone is a dumb phone, Pete.
I do my serious surfing on my laptop, but appear to have the same problem as you on my tablet.
I do my serious surfing on my laptop, but appear to have the same problem as you on my tablet.
On opening the thread on my tablet, I have to press the 'double right pointing arrow' symbol to take me to the last page.
Nice quick intro to QM
I was doing fine until I reached the "Six Postulates"! 😵
- Status
- Not open for further replies.
- Home
- Member Areas
- The Lounge
- What is the Universe expanding into..