I wanna know if it's possible to charge a cellphone battery (3.6V Ni-MH)
directly from the 3.6V output of a standard (3.6Voutput) battery charger?,
or will i just cause more harm to the battery.
reason for asking: in need of project running off a Cellphone battery
(which must be charged without popping the battery back into the cellphone for recharging).
Has anyone came accross a project similar to this?
directly from the 3.6V output of a standard (3.6Voutput) battery charger?,
or will i just cause more harm to the battery.
- i've measured the output from the charger which actually turned out to be 9v and not 3.6v !!
is this annything to be concerned of? - Also should the max charge current be of any concern
- and lastly, how whould i know once battery is sufficiently charged,
since there wont be any DISPLAY unit to confirm this.
reason for asking: in need of project running off a Cellphone battery
(which must be charged without popping the battery back into the cellphone for recharging).
Has anyone came accross a project similar to this?
Attachments
DON'T charge the battery directly....
While I'm not sure it wuold do damage (because the voltage usually will drop as the battery is charged and will be less than 9V), don't try it!
It could be hazardous. The battery and charger may overheat!!
The battery may even explode.
Ni-MH batteries should be recharged with a constant current (usually) and knowing the right capacity of the battery.
If you want I can further guide you... but I think you had better using another kind of battery.... I would use three AA cells...
While I'm not sure it wuold do damage (because the voltage usually will drop as the battery is charged and will be less than 9V), don't try it!
It could be hazardous. The battery and charger may overheat!!
The battery may even explode.
Ni-MH batteries should be recharged with a constant current (usually) and knowing the right capacity of the battery.
If you want I can further guide you... but I think you had better using another kind of battery.... I would use three AA cells...
seeing as that is to dangerous...
my origional intension was to create a similar project as this.
snoop
But these IC's are either extremely expensive or not in stock. Since the 1.5v cells are easilly come by (as well as their chargers) ,
but not the IC's, I thought i'd improvise...
my origional intension was to create a similar project as this.
snoop
But these IC's are either extremely expensive or not in stock. Since the 1.5v cells are easilly come by (as well as their chargers) ,
but not the IC's, I thought i'd improvise...
To properly charge a cell phone battery or any Lithium Ion battery in general you need to use a Constant Current Constant Voltage charge method. You need to have a tightly controlled 4.1-4.2V output voltage and limit the current to less than 1C rate, preferably around 0.7C. On a 1000mAh cell, 1C would be 1000mA, 0.7C is 700mA.
To determine end of charge you wait for the charge current in constant voltage mode to drop below a specified level. Most cell phones have 50mA as the end of charge detection.
Too much current, too much voltage, high temps, and other malfunctions can cause a lithium Ion battery to explode. Cell phones have extensive circuitry to monitor all the parameters to make sure charging is done properly.
To determine end of charge you wait for the charge current in constant voltage mode to drop below a specified level. Most cell phones have 50mA as the end of charge detection.
Too much current, too much voltage, high temps, and other malfunctions can cause a lithium Ion battery to explode. Cell phones have extensive circuitry to monitor all the parameters to make sure charging is done properly.
Cell phones have extensive circuitry to monitor all the parameters to make sure charging is done properly.
Most of the time,I have found that this circuitry (the "watchdog" as I guess it is called.) is inside the battery pack.
On most of the NiMh and all of the Li-Ion packs I've taken apart,the "watchdog" was inside the battery part. If you were to take one apart,there would be the cell(s) and a small circuit board with a couple SMD IC's and whatnot on it -this monitors the charge and discharge cycles,usually also has have overcurrent protection (incase you short the output,as I did.
 -it 'reset' about 10 seconds later. DO NOT short the battery by itself!) and low-voltage cut-off,and other features..(the 'battery meter',for instance.)
-it 'reset' about 10 seconds later. DO NOT short the battery by itself!) and low-voltage cut-off,and other features..(the 'battery meter',for instance.) Note there's usually atleast 3 or 4 pin connectors on Cellphone/laptop battery packs.
It is possible to hack them,I've done it several times (both cellphone batteries,and laptop batteries)..
But DO be careful!

Read up about the proper charging methods,etc..
Don't overcharge them,or short them out,etc.
Exploding batteries can really Fubar your day.

Cell phone battery packs will have the safety circuitry inside, to protect form over voltage, undervoltage, and over current. But in the vast majority of cases the charge control, fuel gauging and secondary safety monitoring is done inside the phone itself. It costs too much for a manufacturer to add an IC to handle those things inside the battery pack when you can use the power management IC inside the phone to handle those chores. When I referred to the monitoring done in the phone I was speaking of actually monitoring and controlling the charge current and voltage, making sure that the battery temperature is in range, having timers to make sure charging doesn't continue for too long, etc.
5 or so years ago "smart" batteries were more common than they are today. Back then you would have 1 or 2 pins used for some sort of serial communication between the battery pack and the phone. Now the extra pins on those batteries are used primarily for a thermistor for the phone to know the temperature of the battery pack.
5 or so years ago "smart" batteries were more common than they are today. Back then you would have 1 or 2 pins used for some sort of serial communication between the battery pack and the phone. Now the extra pins on those batteries are used primarily for a thermistor for the phone to know the temperature of the battery pack.
firstly, Thanks for all your input sofar...
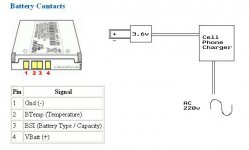
iv'e measured the i/p Charger Voltage = 8.3V
o/p pins of phone to battery = 3.7V
so nokia does provide some kind of DC/DC converter before
giving power to battery pins, but...
----------------------------------------------------------------
For Cell outputs to battery
pin1 (gnd) = 3.71V
pin2 (Btemp)= 2.18V
pin3 (BSI) = 0.92V
pin4 (+) = 3.7V
----------------------------------------------------------------
On Battery itself [all wrt pin4 (+)]:
pin1 (gnd) = 3.71V
pin2 (Btemp)= 3.69V
pin3 (BSI) = 3.70 to 3.71V
thus showing there is voltages to be taken into concern from pins other than pin1&pin4.
So how can one then "hack" the battery to be able to use it with a homemade project charger?
I think the following image will give some more light on my idea.
iv'e measured the i/p Charger Voltage = 8.3V
o/p pins of phone to battery = 3.7V
so nokia does provide some kind of DC/DC converter before
giving power to battery pins, but...
----------------------------------------------------------------
For Cell outputs to battery
pin1 (gnd) = 3.71V
pin2 (Btemp)= 2.18V
pin3 (BSI) = 0.92V
pin4 (+) = 3.7V
----------------------------------------------------------------
On Battery itself [all wrt pin4 (+)]:
pin1 (gnd) = 3.71V
pin2 (Btemp)= 3.69V
pin3 (BSI) = 3.70 to 3.71V
thus showing there is voltages to be taken into concern from pins other than pin1&pin4.
So how can one then "hack" the battery to be able to use it with a homemade project charger?
I think the following image will give some more light on my idea.
Attachments
Pins 2 & 3 are used by the phone to monitor the battery temp most likely. There is usually a thermistor in the battery pack that the phone sends a constant current through, and measures with an A/D on the power management IC.
You can do what is done in the picture by setting a power supply to 4.2V with a current limit of say 0.7A and you will get the Constant current constant voltage you need. In most cases you will be OK doing this. But you don't get some of the backup monitoring you would with a real charging circuit such as watchdog timers (if the battery doesn't reach the current cutoff within say 3 hours using a 1C CCCV charge it could indicate that internal shorts have developed in the cell). Also you don't have any type of temperature monitoring, a Li-Ion cell should only be charged between 0-45C, above this you can damage the battery's cycle life and at higher temperatures can lead to fire or explosions.
You can do what is done in the picture by setting a power supply to 4.2V with a current limit of say 0.7A and you will get the Constant current constant voltage you need. In most cases you will be OK doing this. But you don't get some of the backup monitoring you would with a real charging circuit such as watchdog timers (if the battery doesn't reach the current cutoff within say 3 hours using a 1C CCCV charge it could indicate that internal shorts have developed in the cell). Also you don't have any type of temperature monitoring, a Li-Ion cell should only be charged between 0-45C, above this you can damage the battery's cycle life and at higher temperatures can lead to fire or explosions.
on that note, i could use a Volatge/Current regulator with set outputs , and it will charge nicely.
but the problem is knowing when the battery is fully charged,
since the regulator will just keep providing power regardless of whether the battery is fully charged or not, unless you have some kind of comparator cct (win some/loose some )
)
So does this mean it's safe to strap on a 3.7v - 4.2v supply without worrying too much about charge time??
I just fear it doesn't OVERCHARGE !!
!!
Sorry for bad link, here it is again,similar project:
but the problem is knowing when the battery is fully charged,
since the regulator will just keep providing power regardless of whether the battery is fully charged or not, unless you have some kind of comparator cct (win some/loose some
 )
)Cell phone battery packs will have the safety circuitry inside, to protect form over voltage, undervoltage, and over current. But in the vast majority of cases the charge control, fuel gauging and secondary safety monitoring is done inside the phone itself. -jc2
So does this mean it's safe to strap on a 3.7v - 4.2v supply without worrying too much about charge time??
I just fear it doesn't OVERCHARGE
 !!
!!Sorry for bad link, here it is again,similar project:
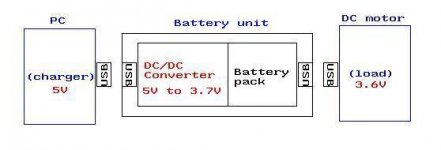
i need it to work like a stand-alone unit, being able to be charged via pc USB-5V; or car supply-12V
(remember, it will have a DC/DC regulator/converter unit built into the project unit), so car just needs a basic USB connector port.
so basically, it musn't be confined to Cellphone applications only!
should be a versitile device. I'm sure you'd love a tiny device that is "hotpluggable," that can be used in a much broader field,
than just cellphones, eg MP3 players, PSP's and many more...
(remember, it will have a DC/DC regulator/converter unit built into the project unit), so car just needs a basic USB connector port.
so basically, it musn't be confined to Cellphone applications only!
should be a versitile device. I'm sure you'd love a tiny device that is "hotpluggable," that can be used in a much broader field,
than just cellphones, eg MP3 players, PSP's and many more...
There are many Li-Ion charge ICs available that would do a better job than just using a regulator. Most will have a built in comparator to stop the charging, and a way to set the current properly with an external resistor or something, and use a pass transistor (internal or external) to do the voltage and current regulation. Some might even have temperature montioring as well.
If you are building this into a device, I would go this route, then you know that the charging is handled properly.
National, Maxim, TI, and others all have solutions, some include charging from USB as the main function.
If you are building this into a device, I would go this route, then you know that the charging is handled properly.
National, Maxim, TI, and others all have solutions, some include charging from USB as the main function.
Ok, you seem to be rather sure you must use this strange kind of battery as yuor power source.... 
So let's see what is the safe, easy and costless way to recharge it....
1) Check the battery capacity:
a) charge it with a phone until it is fully charged;
b) discharge it through a 33 ohm 1 watt resistor while monitoring battery voltage
c) measure the time needed to reach 2,7 V
d) calculate the capacity: T * 100 mA, where T is the time needed in hours; you get the capacity in mAh. (It should be between 500 and 1000 mA)
2) The best, inexpensive and safe (but slow) way to charge a Ni-MH battery is to use 14 hours of constant current using a current which is 1/10 the capacity of the battery e.g. 100mA for a battery of 1000mAh.
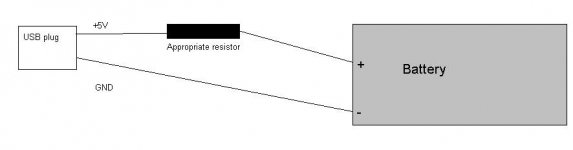
You should need a regulator to get constant current, but this makes the circuit complex and you even have the problem of only 5 V coming from USB...
But the solution is to use a resistor which regulates the current quite well (at least more than enough for your purpose).
The resistor should be choosen according to the formula:
Suppose you need a charging current of 100 mA (0,1 A):
resistor = 1,1 V / 0,1 A
I reccomend a 1 watt resistor
Connect the +5V USB pin to the battery + through the resistor. and GND to -.
Don't use the other pins. Recharge for 14hours only with almost empty battery.
Don't recharge too long.... but a little bit of overcharging is not dangerous with such a safe way to recharge.
Be sure the battery is Ni-MH and NOT Lithium!!
Ask me more if you need!

So let's see what is the safe, easy and costless way to recharge it....
1) Check the battery capacity:
a) charge it with a phone until it is fully charged;
b) discharge it through a 33 ohm 1 watt resistor while monitoring battery voltage
c) measure the time needed to reach 2,7 V
d) calculate the capacity: T * 100 mA, where T is the time needed in hours; you get the capacity in mAh. (It should be between 500 and 1000 mA)
2) The best, inexpensive and safe (but slow) way to charge a Ni-MH battery is to use 14 hours of constant current using a current which is 1/10 the capacity of the battery e.g. 100mA for a battery of 1000mAh.
You should need a regulator to get constant current, but this makes the circuit complex and you even have the problem of only 5 V coming from USB...
But the solution is to use a resistor which regulates the current quite well (at least more than enough for your purpose).
The resistor should be choosen according to the formula:
Suppose you need a charging current of 100 mA (0,1 A):
resistor = 1,1 V / 0,1 A
I reccomend a 1 watt resistor
Connect the +5V USB pin to the battery + through the resistor. and GND to -.
Don't use the other pins. Recharge for 14hours only with almost empty battery.
Don't recharge too long.... but a little bit of overcharging is not dangerous with such a safe way to recharge.
Be sure the battery is Ni-MH and NOT Lithium!!
Ask me more if you need!
Attachments
whooo.....that got me hooked to the screen for some time.
I'm just going through some products from National right now that has to do with this project.
Just have a look at this in the mean time...
Simple Rechargable Battery
I'm just going through some products from National right now that has to do with this project.
Just have a look at this in the mean time...
Simple Rechargable Battery
Sorry, I missed that you said Ni-MH in the first post, since most cell phone batteries in the past 5 years have been lithium Ion. If it is indeed Ni-MH, then the method written above will work just fine. Though if it is an old battery I would be wary of how much capacity it still has.
youre 100% correct. The project will be making use of only "Ni-MH" type cells. the link was just interesting reading material that I thought has great relevance to ours, since the charging method is very simular to what has been discussed.
does this then state that both Ni-cad & Ni-mh make use of the same charging setup?
I checked the specs for my Ni-MH battery:NiMH 900 mAh (BMC-3)
but, where do you get the 1,1v from?
does this then state that both Ni-cad & Ni-mh make use of the same charging setup?
I checked the specs for my Ni-MH battery:NiMH 900 mAh (BMC-3)
but, where do you get the 1,1v from?
resistor = 1,1 V / 0,1 A
Yes, Ni-Cd and Ni-MH can be both charged with the method above.
If your battery is new then you can be pretty sure that the capacity is 900 mAh.... but if it is very old.... It would be better to measure....
1,1 V comes simply from the fact that USB supplies 5 V and charging Ni-MH with the specified method cause the battery to reach about 3,9 V.... So 5 V - 3,9 V = 1,1 V this is what is left to recharge the battery.
This figures are not very precise but they accomplish the task reliably.
OK?
If your battery is new then you can be pretty sure that the capacity is 900 mAh.... but if it is very old.... It would be better to measure....
1,1 V comes simply from the fact that USB supplies 5 V and charging Ni-MH with the specified method cause the battery to reach about 3,9 V.... So 5 V - 3,9 V = 1,1 V this is what is left to recharge the battery.
This figures are not very precise but they accomplish the task reliably.
OK?
- Status
- This old topic is closed. If you want to reopen this topic, contact a moderator using the "Report Post" button.
- Home
- Design & Build
- Parts
- Ingeneous Cell Battery charger