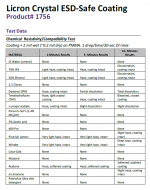
Licron .....holds very little to no charge (when I discharge the membrane to ground there is next to nothing) Jos Wouters
Jos - Very interesting findings thanks. I wonder how you found the graphite coating behaves in holding charge compared to the Licron?
More generally how does charge density on the membrane affect the sound? Anyone?
Hi
Seems like a high carbon loading.
So ending up with 1/3 of Mylars density is unlikely.
Does your coating feel smooth? Or is it more like paper?
There must be an explanation for having density way too small.
Anyway if you enjoy the sound it actually doesnt matter ofcourse
Seems like a high carbon loading.
So ending up with 1/3 of Mylars density is unlikely.
Does your coating feel smooth? Or is it more like paper?
There must be an explanation for having density way too small.
Anyway if you enjoy the sound it actually doesnt matter ofcourse
Jos - Very interesting findings thanks. I wonder how you found the graphite coating behaves in holding charge compared to the Licron?
More generally how does charge density on the membrane affect the sound? Anyone?
Hi Kazap,
I tested it by charging the membrane by connecting it to the EHT board and than discharge to ground through a high voltage probe and check the time that it takes to get to zero on the multimeter. No idea if this is a reliable way of testing it, but with this test it took the graphite coating much longer to discharge than it took the Licron coating. Also if you discharge the membrane before working on the ESL the Licron shows and hears no sparking of discharge at all, where the graphite coating sparks a lot and makes a hissing sound for a second or so when touching the contacts. But, maybe less is better and more charge is worse? How much charge is enough? And again my question how to measure that charge?
Would be nice if anyone could answer your question about charge. Before the how question there is the question IF the charge density affects the sound? Or do other elements (also) influence that? I have no idea. Other influencers can be: surface structure, density, chemical structure, and maybe many more.
I have until now not been able to find an affordable meter that can measure (dis)charge in coulomb at 5000V and see the polarity. Most cheap coulomb meters are 100V or less and are therefore of no use for me.
Unlikely, not plausible. Call it what it is. As I said before the measurements can be off by 10% because of cheap meters, but will not be of by 100%-300%. Why 3 micron coating weighs only 1 micron Mylar I don't know and I don't care. I just read my meter and scale. I can't make any interpretations on it even if I would, because I do not have the knowledge nor the facilities to give it any value.Hi
Seems like a high carbon loading.
So ending up with 1/3 of Mylars density is unlikely.
Does your coating feel smooth? Or is it more like paper?
There must be an explanation for having density way too small.
Anyway if you enjoy the sound it actually doesn't matter of course
Smooth? that is a subjective question. I have no ways of measuring how rough or smooth the surface is in comparison to Mylar. How open or closed the coating is? I looked at the coating with 1600X magnifying microscope and that still tells me nothing. And again if I had that number what would it tell? If I said it feels pretty smooth of quite rough. Again subjective and of no value.
I like this discussion a lot because it looks a lot like my DIY project on the Quad ESL-63 the last 2 years, many many more question than answers. Beautiful challenges. And with every answer 10 new questions pop up.
Hope there are people on this forum who can chip in. Keep challenging me and maybe my mind will come up with new ways of looking at the problems at hand. Please remember there are no wrong answers or bad remarks. Anything can help. Thanks in advance.
Last edited:
Jos - Very interesting findings thanks. I wonder how you found the graphite coating behaves in holding charge compared to the Licron?
More generally how does charge density on the membrane affect the sound? Anyone?
Charge on the mylar is a function of capacity and voltage only. C=Q/V
IMO This formula is applicable for a capacitor not for an ESL.Charge on the mylar is a function of capacity and voltage only. C=Q/V
IMO This formula is applicable for a capacitor not for an ESL.
An ESL is a capacitive element. So this rule applies.
There are no miracle coatings having more charge than other coatings.
@wout31: ... A thought popped up: Could it be that somehow your coating formula - or the process you use - causes the coating to "foam"? I.e. it will have low weight, yet be relatively thick due to "micro-bubbles" inside the coating?
Cheers,
Jesper
These small bubbles would resemble extreme surface tension energy in order to exist.
A bubbled coating solution also decreases in volume after time, maybe Jos observed this behaviour, but again it is highly unlikely because of the surface tension.
More likely to be a dust particle, uneven coating, error of measurement etc etc etc
Last edited:
You lost me here.These small bubbles would resemble extreme surface tension energy in order to exist.
A bubbled coating solution also decreases in volume after time, maybe Jos observed this behaviour, but again it is highly unlikely because of the surface tension.
More likely to be a dust particle, uneven coating, error of measurement etc etc etc
It might.@wout31: ... A thought popped up: Could it be that somehow your coating formula - or the process you use - causes the coating to "foam"? I.e. it will have low weight, yet be relatively thick due to "micro-bubbles" inside the coating?
Cheers,
Jesper
That was my experience with Licron as well. Resistance did not change with humidity level, but moisture on the surface did increase conductivity. A few minutes under a fan and conductivity returned to reference level. I found Licron to be rather robust, rubbing and cleaning with soap and water had no effect on the conductivity. It is also resistance to ozone, which is a good thing for ESLs. The only shortcoming I found was lack of UV resistance. Direct sunlight will slowly reduce the coating conductivity over time…eventually requiring recoating.About your licron experiment; you lose resistance? Well I think you are increasing conductivity by adding moisture which I think is a normal thing to happen. Remember your wet finger also contains dissolved salts etc...
If for some reason you do need to remove a Licron coating, I found acetone on an absorbent pad to work well.
Attachments
wout31 Interesting results. I also agree that there might be an error hidden somewhere, regarding wheight and thickness measurement. And I guess you are clever enough to find that out...
Your coating looks very close to the original, and thats nice. And I have to say your findings are more interesting than watching the ballgame on TV!!
Your coating looks very close to the original, and thats nice. And I have to say your findings are more interesting than watching the ballgame on TV!!
Took a further look at the coating and any possible errors in the numbers, thickness and weight.
Density of Mylar is 1.4
Density of graphite coating is 0,8 - 0,7 (very close calculated approximation as several components are involved)
Particle size of the graphite (as by specification of the supplier of graphite powder) 3 micron. So it is correct that the thickness can be no less than 3 micron, as that is the particle size. I measure 3-4 micron thickness.
Density of Mylar is bigger, of the coating is smaller, so the coating is lighter.
Because the graphite particles are 3 micron it might be that the rest of the coating applied is thinner and I measure the top of the coating (the highest points).
That again can explain less weight as maybe not the whole of the coating layer is 3 micron thick.
At Alibaba I found a graphite powder supplier that can supply graphite as finer powder with particle size of 1 micron. To be able to test I have to be able to buy it in small quantity at a reasonable price. Minimum orders at the suppliers I found are 100 kg or 1 ton :-(
Can't find any mistakes here. Are my conclusions wrong? I have applied several coatings the last weeks and all come to the same result in thickness and weight. Always the same numbers.
Density of Mylar is 1.4
Density of graphite coating is 0,8 - 0,7 (very close calculated approximation as several components are involved)
Particle size of the graphite (as by specification of the supplier of graphite powder) 3 micron. So it is correct that the thickness can be no less than 3 micron, as that is the particle size. I measure 3-4 micron thickness.
Density of Mylar is bigger, of the coating is smaller, so the coating is lighter.
Because the graphite particles are 3 micron it might be that the rest of the coating applied is thinner and I measure the top of the coating (the highest points).
That again can explain less weight as maybe not the whole of the coating layer is 3 micron thick.
At Alibaba I found a graphite powder supplier that can supply graphite as finer powder with particle size of 1 micron. To be able to test I have to be able to buy it in small quantity at a reasonable price. Minimum orders at the suppliers I found are 100 kg or 1 ton :-(
Can't find any mistakes here. Are my conclusions wrong? I have applied several coatings the last weeks and all come to the same result in thickness and weight. Always the same numbers.
Last edited:
To finish my thoughts and calculation.
Density Mylar 1.4 x 3 (micron) = 4.2
Density coating 0.75 (averaged calculated density) x 2 micron (assumed coating average thickness, can't verify that but see the explanation above) = 1.5
1.5 / 4.2 = 0.35
So IMO the weight of the coating can be substantially less of that of the Mylar with the measured thickness of 3-4 micron
Density Mylar 1.4 x 3 (micron) = 4.2
Density coating 0.75 (averaged calculated density) x 2 micron (assumed coating average thickness, can't verify that but see the explanation above) = 1.5
1.5 / 4.2 = 0.35
So IMO the weight of the coating can be substantially less of that of the Mylar with the measured thickness of 3-4 micron
An externally hosted image should be here but it was not working when we last tested it.
This is the instrument that puts on the high pressure so it gives me 300% error reading.
I will send it back to manufacturer to get me refund.
Last edited:
Thats a cool looking device....
Do you apply some grinding process for making coating? Or do you rub it afterwards?
I ask because if your conductive particles are really 3 micron and you need at least 2 of them stacked on eachother to make a conductive network, there is (with or without compression) no chance to get thinner than 6 micron (being highly optimistic).
Do you apply some grinding process for making coating? Or do you rub it afterwards?
I ask because if your conductive particles are really 3 micron and you need at least 2 of them stacked on eachother to make a conductive network, there is (with or without compression) no chance to get thinner than 6 micron (being highly optimistic).
- Home
- Loudspeakers
- Planars & Exotics
- ESL Diaphragm coating