For many years I have avoided making my own boards, preferring the sloppy web of components. Perhaps the time has come to try my hand at creating a printed circuit board.
Can anyone offer suggestions as to how to proceed? Money is, of course, a major consideration so I want to keep my investment small. I only want to make small stuff, say an op amp with a few transistors and passive components.
Alternatively, is there a breadboarding scheme that isn't clumsy and can let me build a few little things?
If you are used to using through-hole components you might like using vector board. The component leads themselves become your traces which obviates the need for chemicals. It gives you almost unlimited flexibility in playing around with your layout before soldering and is a good way to teach yourself layout for when you get into PCBs. To prevent waste I do my layout on an uncut piece first and mark positions then score and break the board to suit. You can get very tight layouts when you need them this way too.
I feel like a salesman.
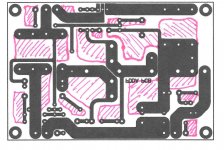
pcb Design
My question: why would you not leave the extra copper as shown in the pink. It doesn't change layout, connections, etc. just more copper left on board.
Thanks for helping me understand this!
I hope this is not considered 'off topic' if so, I'll start a new thread. Anyway, I'm just learning the elements of designing pcb's and it seems as quoted above that the more copper you leave on the board, the better - for all kinds of reasons. So, here is a professionally designed board (Nelson Pass) with my modifications in pink. Now, for a newbie to modify the design of a Master might be pretty arrogant, but I've always thought you should learn from/steal from the best.. In hindsight, I would try harder to have more PCB surface area covered in copper so there's less to remove, to require less etchant.
My question: why would you not leave the extra copper as shown in the pink. It doesn't change layout, connections, etc. just more copper left on board.
Thanks for helping me understand this!
Attachments
Brother printers don't work well
Others have said it, and it's really true-- Brother brand printers don't work well for toner-transfer method.
I am etching boards again now, with the same process I used before except with a new printer. It's a Brother and it didn't give good adhesion. The result is a board where the copper areas are thoroughly pock-marked by etchant that got through the holes in the etch mask. It looks like the surface of the moon
What was the original printer that worked better? It was whatever my old office had, maybe a Xerox or a Lexmark... I've left that job and don't remember its identity, sorry.
Others have said it, and it's really true-- Brother brand printers don't work well for toner-transfer method.
I am etching boards again now, with the same process I used before except with a new printer. It's a Brother and it didn't give good adhesion. The result is a board where the copper areas are thoroughly pock-marked by etchant that got through the holes in the etch mask. It looks like the surface of the moon
What was the original printer that worked better? It was whatever my old office had, maybe a Xerox or a Lexmark... I've left that job and don't remember its identity, sorry.
old hp laserjets (like the 4P) historically work well, especially using original hp toner.
there are some sites that discuss this but i have no links at the moment ...
mlloyd1
there are some sites that discuss this but i have no links at the moment ...
mlloyd1
environmentally safe etch disposal
I don't know what I'm doing, but this chemistry stuff is fun.
My understanding (and I welcome correction) is that to dispose of used etchant properly, you have three options:
1- Get the copper to precipitate out. Insoluble copper salts are safer than soluble ones, they can be safely thrown away.
2- Evaporate off the etchant, leaving crystals of soluble copper salts. These can be burned, which will oxidize everything and leave behind nothing too reactive.
3- Take it to the authorities on household hazardous waste disposal day and let them deal with it. (But what do they do with it? Inquiring minds want to know!)
I've been using the salt + peroxide + vinegar recipe to etch. This leaves behind a clear blue liquid and no precipitate. That would be nasty soluble copper salts. Do not drink.
Evaporation would surely work, but it would take a long time and I have to find a secure place where kids and dogs won't get into the stuff.
What I've hit on is a two part disposal process:
1- React the stuff with an excess of baking soda to make it smell better (not like vinegar) and be less acidic. Baking soda is a weak base.
2- React the stuff with some aluminum foil, as suggested here and elsewhere:
Simple PCB etchant made from chemicals you can put in your mouth | Hackaday
That fizzes slowly until it doesn't, and makes some precipitate. After this step, you can filter the etchant through a coffee filter to remove the precipitate. The remaining liquid is clear (or VERY close) so I think most of the copper has been removed! Most soluble copper compounds I know about turn the water green or blue or turquoise, none of them are clear. (Again I welcome correction, I don't know what I'm talking about.)
The hackaday commenter suggests doing these steps in reverse order-- aluminum first, then baking soda. Maybe next time I'll try that. Or maybe next time I'll try one of the reusable etchant recipes, disposing of this stuff properly is a pain in the butt!
I don't know what I'm doing, but this chemistry stuff is fun.
My understanding (and I welcome correction) is that to dispose of used etchant properly, you have three options:
1- Get the copper to precipitate out. Insoluble copper salts are safer than soluble ones, they can be safely thrown away.
2- Evaporate off the etchant, leaving crystals of soluble copper salts. These can be burned, which will oxidize everything and leave behind nothing too reactive.
3- Take it to the authorities on household hazardous waste disposal day and let them deal with it. (But what do they do with it? Inquiring minds want to know!)
I've been using the salt + peroxide + vinegar recipe to etch. This leaves behind a clear blue liquid and no precipitate. That would be nasty soluble copper salts. Do not drink.
Evaporation would surely work, but it would take a long time and I have to find a secure place where kids and dogs won't get into the stuff.
What I've hit on is a two part disposal process:
1- React the stuff with an excess of baking soda to make it smell better (not like vinegar) and be less acidic. Baking soda is a weak base.
2- React the stuff with some aluminum foil, as suggested here and elsewhere:
Simple PCB etchant made from chemicals you can put in your mouth | Hackaday
That fizzes slowly until it doesn't, and makes some precipitate. After this step, you can filter the etchant through a coffee filter to remove the precipitate. The remaining liquid is clear (or VERY close) so I think most of the copper has been removed! Most soluble copper compounds I know about turn the water green or blue or turquoise, none of them are clear. (Again I welcome correction, I don't know what I'm talking about.)
The hackaday commenter suggests doing these steps in reverse order-- aluminum first, then baking soda. Maybe next time I'll try that. Or maybe next time I'll try one of the reusable etchant recipes, disposing of this stuff properly is a pain in the butt!
- Status
- This old topic is closed. If you want to reopen this topic, contact a moderator using the "Report Post" button.
- Home
- Design & Build
- Construction Tips
- Making my own PC boards